Efficacy data
PrKORSUVA® reduced itch intensity (≥3-point improvement in WI-NRS) at Week 12 from baseline vs. placebo1†s
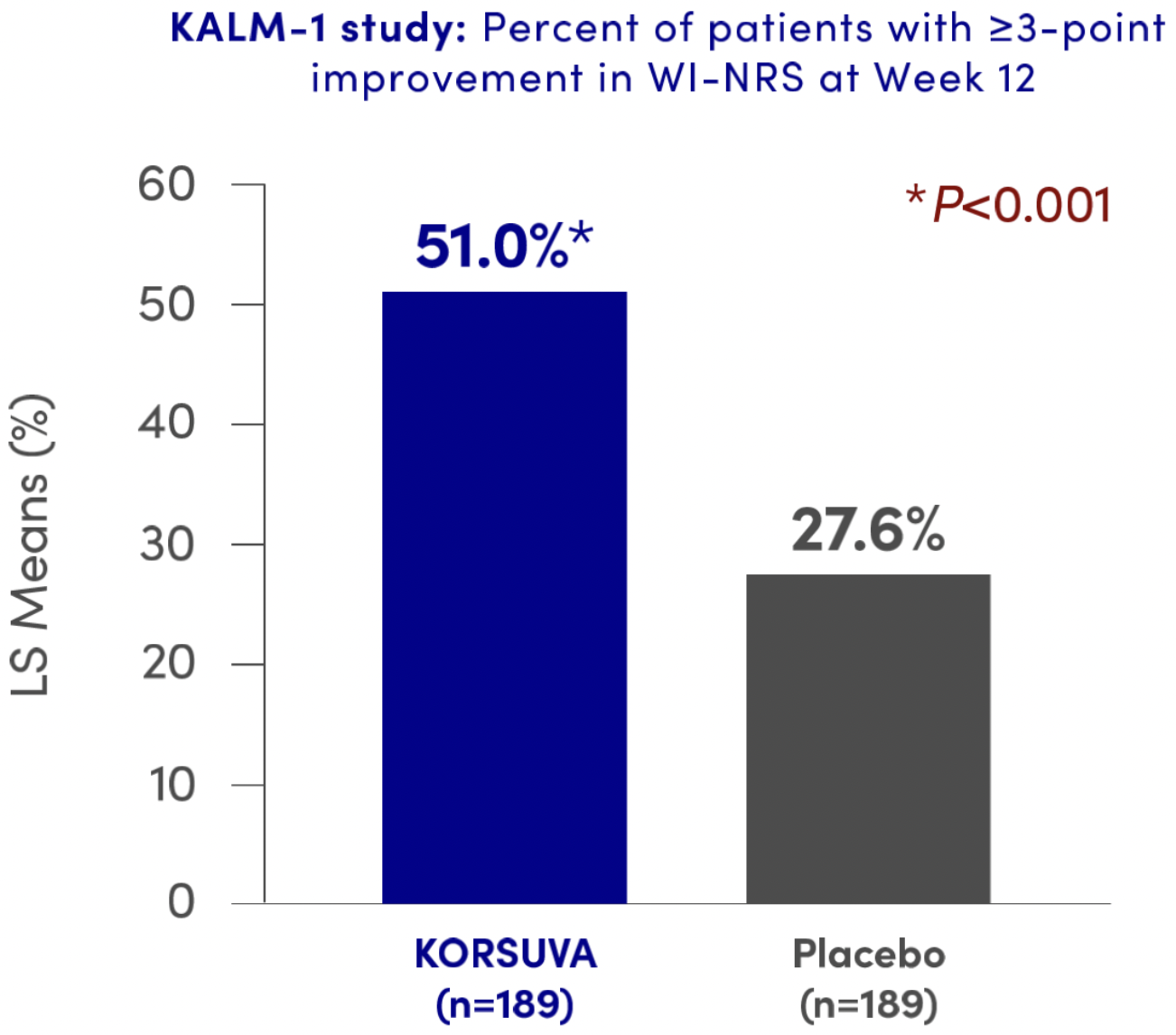
Number observed with ≥3-point NRS improvement: 157 (Yes=82, No=75; missing n=32) and 165 (Yes=51, No=114; missing n=24) for KORSUVA and placebo, respectively. Counts were based on non-missing data.
Estimated percent and P-value used a logistic regression model with terms for treatment group, baseline Worst Itching Intensity numerical rating scale score, use of anti-itch medication during the week prior to randomization, and the presence of specific medical conditions. Missing values were imputed using multiple imputation under missing-at-random missing data assumption for interim patients and post-interim patients separately.
Adapted from the Product Monograph.1
Demonstrated results for itch-related QoL (Skindex-10 and 5-D Itch scores) at Week 12 (secondary endpoints)1†
In KALM 2, Skindex-10 and 5-D Itch scores did not reach statistical significance.


Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.

